By: Nicole Todd and Roma Parikh

Our literature in the library class gets up to many surprising and challenging tasks throughout the semester, which Dr. Camp has blogged about before. One of these projects was chemically analyzing one of the medieval fragments in the Special Collections library. We were presented with the task of hypothesizing what pigments were used in the making of the manuscript and then analyzing scientific data to help prove or disprove those hypotheses. Our manuscript fragment contains parts of the Latin Book of Tobit, and was made in Paris, France in the 1300s. When we first started our analysis of our manuscript fragment, we did not expect to find any shocking or mysterious information, because our fragment was relatively simple. It only has three colors: black, red, and blue. There are no illuminations and only three decorated initials. Based off information about the manuscript such as when it was made and where, along with research about commonly used pigments for the period, we made our hypotheses. Still, it was not as exciting or fascinating as some of the other fragment analysis. Or so we thought…
For the pigment analysis, the instrument that we used to base our information on was a portable X-Ray fluorescence spectrometer, otherwise referred to as pXRF. This instrument was the most logical choice for our evaluation of pigments because it is a non-invasive, portable, and we had an excellent guide, Dr. Hunt, from the Center for Applied Isotypes. While infinitely more complicated a device than we can explain, a pXRF spectrometer generally works by shooting gamma rays at the object, exciting electrons which then emit an energy signal the device is able to measure and correlate to a certain element. Dr. Camp and our fellow classmate, Bonnie, cover this project and the device itself in greater detail in separate blog posts. Luckily, we were able to analyze the results from the pXRF, called spectra with the use of Artax. Artax is a simple software that allowed us to view spectra and helped chart the energy levels and pulses that came from our object. Then, we would be able to identify which element these peaks belonged to. Once we began investigating our results, the spectra, we stumbled upon one of the most interesting aspects of our fragment.
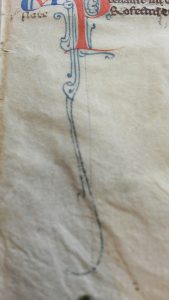

As stated before, we did not expect to find any mind boggling results when interpreted our pXRF results. One of our main questions going into the analysis was whether or not the same blue pigment was used for the decorated ‘I’ initial and the spiralling blue lines at the bottom of the page. The difference in color and appearance is clear from up close images of both as seen here. Given how rich and opaque the blue was, we imagined it might be a nicer pigment, ultramarine, which was created from the rare mineral lapis lazuli, transported all the way from present-day Afghanistan to Paris, France (Feller). The other blue we thought would be azurite, a cheaper and widely used pigment across Europe. Ironically, while the spectra of our blue pigments did cause questions to arise, it was about the presence of an entire unexpected element, rather than the identification of a certain type of pigment.
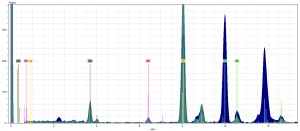
In our pigment analysis of the dark blue and lighter blue, we found that there were high peaks of copper in both samples. The peak of copper is identified by the Yellow marker. The color of the spectra themselves correlate to the color of their pigment in the fragment. The dark blue is the dark blue pigment. The teal is for the blue decorative lines.
The peak of copper indicates that both blues are the pigment azurite, whose chemical formula is 2CuCO3Cu(OH)2 (Feller). Of those elements, only the copper would show up on the spectra analysis, because it would emit enough energy to be accurately identified by the device. We also found in the dark blue spectra a peak of mercury higher than most other element’s peaks in any of our other pigment’s spectra. It is denoted by the turquoise marker. Clearly, it was not a fluke. But this shocked us for a couple of reasons. Only one type of blue contained mercury, despite both obviously being azurite pigments.Secondly, the most logical conclusion for the presence of mercury is that it comes from vermillion, a red pigment which has the chemical formula: HgS, mercury sulfide (Thompson).

If there was a spot of red pigment made from vermillion nearby, that would be the reason. Except for the fact that our red pigment was not actually vermillion. The spectra result for the red pigment showed concentrations of lead and absolutely no mercury. Thus, we concluded our red pigment was red lead, a bright red pigment made by heating up white lead (Feller). If the mercury in the dark blue pigment did not come from a vermillion, then we thought perhaps there was a way to make a blue pigment using mercury.
Researching a mercury blue pigment was tricky, because of how common the mercury red, vermillion was. But undeterred, we made use of the vast print resources available across campus. Dr. Camp has set aside a number of useful resources on course reserve so we could go in and use them for a period of two hours. To our utter frustration, references to mercury were almost universally connected to vermillion or, equally unhelpful, as a base for gilding. Out of the ten to twelve books we scoured through, only one vaguely mentioned something about a mercury blue. It was only a few sentences buried in the middle of the book and at the bottom of the page.
In The Alchemy of Paint: Art, Science and Secrets from the Middle Ages by Spike Bucklow, it mentioned a mercury blue, which would be made from mercury and sulfur with the inclusion of sal ammoniac (to differentiate it from the recipe for vermillion). Furthermore, it included a reference to The Original Treatises on the Arts of Painting by Mary Merrifield. The book was published in 1849, but contained a record of a number of medieval pigment recipes.

Some of these were clearly labelled “To make azure” and included ingredients such as quicksilver, another name for mercury. We were so excited and overwhelmed to think we found the answer to the question of these mysterious mercury peaks.
But we were cautious too. A little thought in the back of our head was warning us it was too good to be true. Perhaps it was the way Bucklow wrote it off as fantasy, “the metallic blues live in the world of thoughts, but have left little or no trace in the world of things” (110). Indeed, a search of chemical analysis of a mercury blue showed that no one had tested for and found a trace of this pigment on their objects. Not that a lack of evidence proved it couldn’t exist or was never used, but it certainly didn’t help. After many several sources, with details of chemistry that went beyond our head, we were coming to the conclusion that mercury blue would not be a sufficient answer (Oltrogge). It was simply not a recipe which seemed capable of producing a blue pigment. One team of researchers even attempted to recreate some of these recipes, but with little success. Some recipes produced a greenish pigment, some a red pigment, and others a black-blue color likely due to oxidization (Orna). Nevertheless, none of those results confirms the existence of a mercury based blue pigment which looked like the blue, rich blue found in our fragment.
While the recipes were intriguing and fanciful, the fact that it has yet to be reproduced, found in other objects, and is simply not chemically possible brings us back to square one. If not mercury blue, what else would cause the mercury found in our dark blue pigments? It shall remain a mystery for now. Personally, we’d like to think we’ve found the first ever documented case of a mercury blue proven with chemical data. Otherwise, until more research comes out or more conclusive tests can be run on this fragment to see what might better explain the source of mercury, can’t we just settle for saying, ‘mercury was in retrograde’? It’s what any good medieval alchemist would have done!
References
Feller, Robert F., editor. Artists’ Pigments: A Handbook of Their History and Characteristics. Vol. 2, National Gallery of Art, 1993. Print.
Orna, Mary V., editor. “Chapter 9: Copper-Based Synthetic Medieval Blue Pigments”, Archaeological Chemistry. ACS Symposium Series. American Chemical Society. Washington, DC. 1996, pp. 107-115. Print.
Oltrogge, Doris. “Recipe Books of Illuminators in 15th Century Germany and Netherlands – Workshop Practice and Encyclopedic Ambition”, Craft Treatises and Handbooks: The Dissemination of Technical Knowledge in the Middle Ages. Turnhout, Brepols. 2013, pp. 61-64. Print.
Thompson, Daniel V. The Materials and Techniques of Medieval Painting. New York : Dover Publications, 1956. Print.