by Eliza Sarazua, Connor Ottem, and Johanna Hoover
Have you ever thought you would get the opportunity to do some insane isotope testing on a manuscript over 600 years old? Neither did we! In our English class titled “Literature in the Library” our class studied and analyzed a french manuscript from the fifteenth-century called the Hargrett Hours. Throughout the Hargrett Hours are double-lined decorated initials that are made with gold. However, some of the initials appear to be faded or scratched away.

Our initial research question was determining whether the faded initials were made with a different substance from the ones that were intact. We questioned if all the initials were made with the same gold and if fading was due to wear and tear. We anticipated testing the gold rinceaux, a decorative pattern of leafy stems, in the decorated borders of the manuscript to determine if we could chemically tell it’s gold. These research questions help us estimate a more exact date for the manuscript’s origin – because of the techniques used for each gold type – and can help us better understand the typical illuminator’s palette during fifteenth-century France.
Shell Gold vs. Leaf Gold
Two major types of gold pigments were typically used in the production of manuscripts: shell gold and leaf gold. In the Hargrett Hours, the leaf gold was used in the decoration of the initials throughout the manuscript (Figure 1).

Shell gold was used in the rinceaux border (Figure 2). The black-outlined rinceaux is complete with drops of gold, while the initials using leaf gold were burnished to the exact shape to create a clear letter form.
If this was gold, we’d expect to find gold without other elements in testing. Sometimes, though, gold leaf appears as an alloy with copper or other metals. This is due to illuminators commonly using the most available gold they could find, not necessarily worried about the purity of the metal. Additionally, because of the many colors around each sampled gold spot, we had to anticipate the elements in the pigments nearby to accurately determine what was in our gold sample.
Chemically, leaf and shell gold are similar, so a physical examination is required to distinguish the two. Leaf gold has a tactile, 3D look and feel to it while shell gold is flat on the page. Shell gold is easier to identify, usually leaking outside of its margin, demonstrating a liquid application and not the solid of the gold leaf, which would be burnished to the exact shape desired. From our physical examination of the Hargrett Hours, we determined the decorated initials were made of gold leaf while the gold designs in the rinceaux were made of shell gold.
In order to analyze the gold in the manuscript, we used a portable X-ray fluorescence (p-XRF) machine to scan the leaves. The machine scans a portion of the leaf and creates a spectrum of the different elements present in the sample. This spectrum is presented as a line graph with elements appearing where there are spikes. Analyzing the rinceaux, for example, may bring up readings of iron and calcium because those make up the inks that link the gold patterns. It’s worth noting that the spectral graphs don’t calculate quantity of an element, but the quality, so a large spike in an element doesn’t mean that there’s a lot of that element present, but that it’s a potent source. The purpose of the spectral analysis then is to determine the quality of gold in the manuscript.
Sample Spots and Spectra
Fol. 18v, Leaf Gold

This sample was taken from fol. 18v. Figure 3 shows the sample on the manuscript page, a gold decorated initial. This initial is mostly intact, though parts are worn away. Figure 4 is the spectrum it produced. The red line represents the initial while the green line represents a sample of the parchment without ink or illumination. We read these samples together to determine what elements are coming from our sample by how much it reaches beyond the ‘standard’ parchment spectra line. From this, we can see that this sample has a high gold reading as well as a calcium reading that is higher than the parchment’s reading. The majority of the calcium is likely from the parchment, though we see that the red peak, our sample spot, goes beyond the green parchment peak. That means that the calcium is getting picked up elsewhere, which we believe is coming from the gesso that was used to seal the gold leaf. The iron and copper readings are most likely from the pigments surrounding the initial, typically used in black and blue inks.
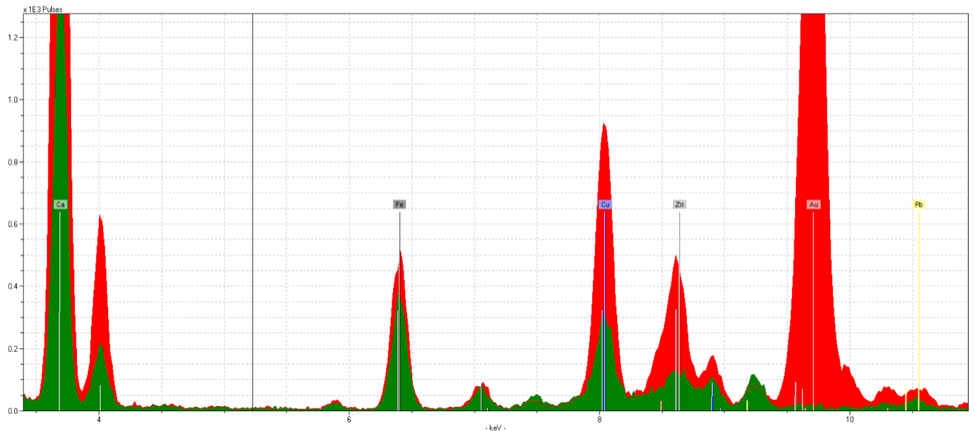
Fol. 19r, Leaf Gold

Figure 5 is from fol. 19r. This initial is noticeably faded, with parts of it appearing grayish in color. However, the spectra presents a strong gold reading. Again, the spectra doesn’t show quantity but rather quality of the element present, and even this faded gold was able to show strong readings. This suggests that the damaged initials aren’t fading due to chemical reasons, but physical. There are other elements found in this sample as well, such as iron, zinc, and copper, all likely to originate from the inks and pigments surrounding the initial.
……………………………………………………………………………………………………………………
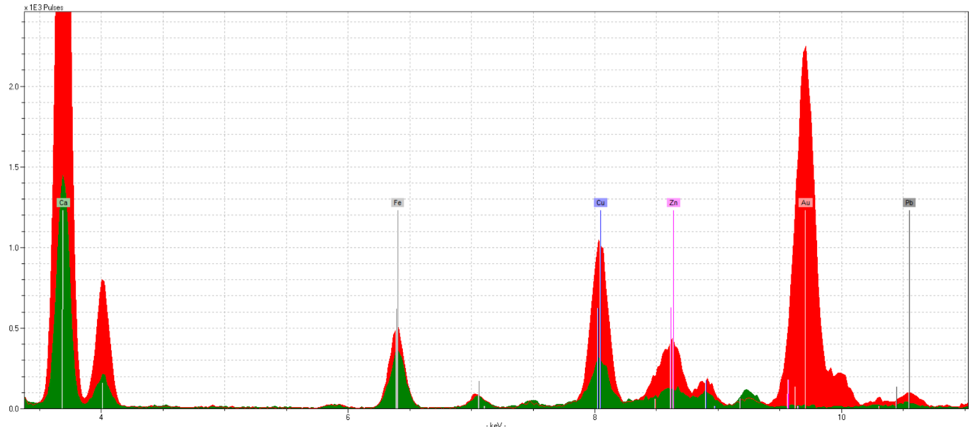
Figure 6: Fol. 19r, Gold Leaf Initial Spectra

Fol. 18v, Shell Gold
Figure 7 is fol.18v, the rinceaux. As expected there’s a strong gold reading, though it is noticeably smaller than the gold readings from the previous samples. The spectra (Figure 8) has a strong iron presence, likely originating from the ink that forms the vine-like pattern between the gold flowers.
……………………………………………………………………………………………………………………
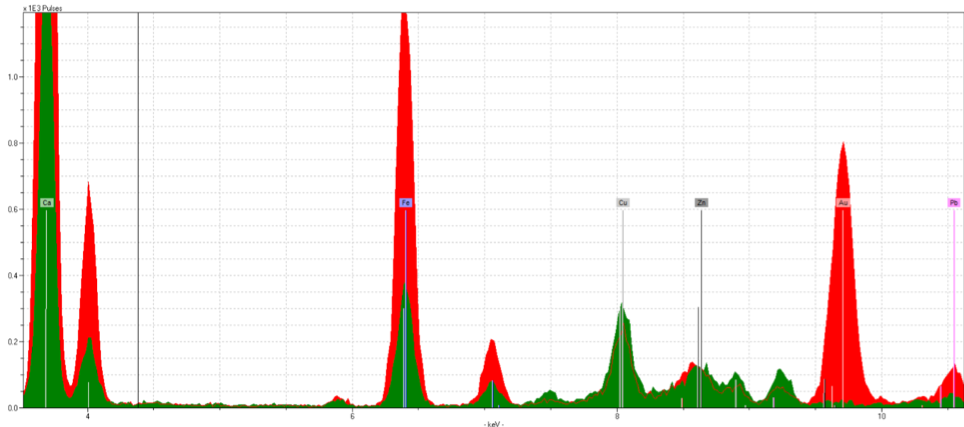
Figure 8: Fol. 18v, Shell Gold Rinceaux Spectra
Conclusion
Through the p-XRF sampling and analysis of our spectra, our hypothesis was unable to be answered by our data. Thus, we couldn’t chemically distinguish what could be causing the fading/peeling seen in the initials throughout the Hargrett Hours. We were also unable to chemically distinguish between the shell gold used in the rinceaux borders and the gold leafing of the initials. Given that the rinceaux is shell gold and in remarkably better condition than the leaf gold initials, this provides insight into the differing durabilities of the two pigments, and that the differences aren’t on a chemical level. Upon further physical analysis, we created a way to help us identify shell gold versus gold leaf. We struggled with what to do since we couldn’t answer our research question with the results we received. One of the hardest aspects of analyzing our spectra was the crowded analysis that came from each sample. Without a clear and isolated spot, we ended up with a lot of ‘noise’ from the surrounding pigments that made determining what elements were coming from the gold in our sample spot – or the pigments around it – rather tedious. We decided that more and different kinds of physical testing is needed to determine why and how some gold initials faded while others didn’t.
