What It’s All About
By Emily Cooley, Corie Bolt, Ceciley Pangburn, and David Walker
Undoubtedly, the oo’s and aah’s of any illuminated manuscript are the decorations and the colors. Let’s be real — unless you can read medieval Latin or French, most of the manuscript is going to be incomprehensible to you. So, we focus on the pictures. Luckily, our research deals with just that: the color. Over the course of a few weeks, we each researched one of the colors present in the calendar of the Hargrett Hours. David Walker took on the black pigments, Emily Cooley opted for the red, Ceciley Pangburn was on the blue, and Corie Bolt went for the gold.
Collectively, we sought to answer the question: is the calendar an original part of the Hargrett Hours?
The calendar appears to have been written by a different scribe than the rest of the manuscript, which could mean that the calendar wasn’t originally written for the Hargrett Hours. However, there is also some evidence that it was. There are feast days in the calendar that are specific to Sainte-Chapelle, a thirteenth-century Parisian chapel. Sainte-Chapelle is famous for having the Crown of Thorns in its collection. This is notable because the rest of the Hargrett Hours has a ton of Passion of Christ related sections, including prayers focused on the Crown of Thorns.
Answering the question about the calendar’s placement in the Hargrett Hours may reveal something about the manuscript’s production and provide another lead for later researchers to follow. We may have gotten some answers, but our research ended up prompting us with an entirely different question:
Where is all this calcium coming from?
The Hypothesis
Our task sounded simple enough: compare the pigments in the calendar to the rest of the Hargrett Hours and see if they matched. So, the first step was to figure out which pigments we expected to find. We were all students coming from different backgrounds with varying amounts of knowledge about medieval manuscripts, but with Dr. Camp’s help and some research, we were able to form a solid hypothesis for each pigment:

Black ink is the most common pigment in the Hargrett Hours. David fully anticipated the black pigment to be iron gall ink, which is made from adding iron sulphate (FeSO4) to tannic acid (C6H2(OH)3COOH).

The red pigment in the calendar was most often used for rubrication. Emily expected to find vermillion (HgS) or red lead (Pb3O4) in her results regarding the red in the calendar. Vermillion was a common pigment used in the 15th century for illuminated manuscripts– but so was red lead, as it was the cheapest way to produce a red color. Since vermillion and red lead are both metal-based, the machine we were using would be able to give us an idea as to whether those pigments were present in the pages we were sampling. Organic reds would be trickier to identify due to limitations in our equipment.

Blue pigments are used for initials in the calendar as decoration. Ceciley took on the blue pigments in the calendar, and anticipated that it would be azurite (Cu3(CO3)2(OH)2). Azurite was a cheaper alternative to ultramarine blue and very commonly used in illuminated manuscripts during this time period.

Corie expected the gold to be pretty straightforward, as gold pigments in this time were limited to only a couple of options and we would be looking for mainly the element of gold on the spectra. A separate group was looking at gold specifically, and their research lays out the several forms of gold commonly used in medieval manuscripts. Corie expected the gold initials to be the work of gold leafing, mostly because of how the initials appeared to be worn thin in some spots, but also because this was pretty common for medieval illuminated manuscripts.
How We Did It
In order to conduct our research, we employed the help of two professionals and a machine specialized for atomic analysis. Of course, Dr. Camp was there to help us throughout all the stages of this experience. Dr. Alice Hunt, formerly an Associate Research Scientist in the Center for Applied Isotope Studies and now with UGA’s Center for Teaching and Learning, helped us with understanding how to read the results from a p-XRF machine as well as answering some questions we had from said results. XRF, or x-ray fluorescence spectroscopy, detects a range of elements with a high mass that are present on the material being tested. Using the program Artax, we were able to analyze the results of the sample spots on the pages we chose. Since our hypothesis requires information about the pigments used in the Hargrett Hours, we needed a way to identify those pigments on an atomic level. After identifying the pigments in the calendar quire, we could see whether or not those pigments differed from those in the rest of the manuscript. This information could provide evidence for either how many scribes worked on the Hargrett Hours or when the different parts of the Hargrett Hours were produced and/or assembled.
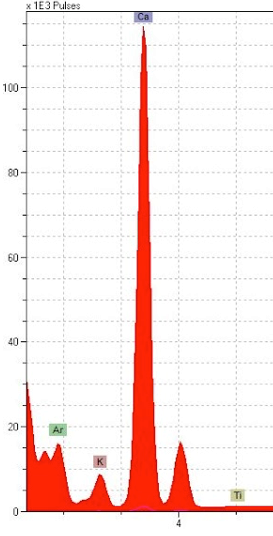
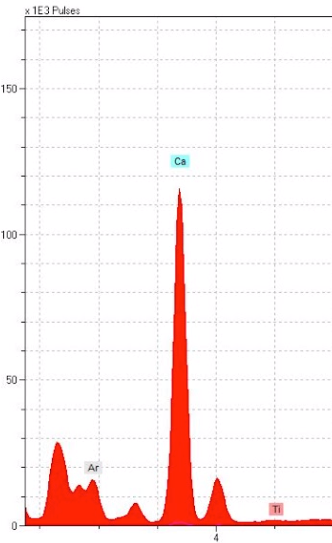
Our results came in the form of “spectra”: ranges of information about the atomic masses of elements present in our sampling spots. “Spectral peaks”, or where the graph had a sudden spike, indicate what particular elements are likely appearing at those spots. The black ink showed a peak of iron that supported our initial hypothesis of iron gall. As expected, our red showed high amounts of mercury, pointing toward vermilion. The blue had high amounts of copper, suggesting azurite. Lastly, the gold had an not unexpected substantial gold spectral peak. So far, the contents of the calendar quire were meeting our expectations and didn’t significantly contradict the findings for the rest of the Hargrett Hours.
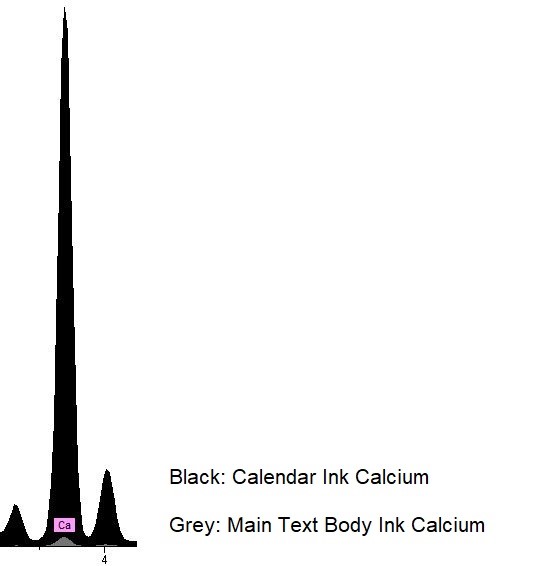
Here comes the curve ball: once we took a look at our p-XRF results, we realized that both the black and the blue pigments had huge spikes of calcium. We had been expecting some calcium because parchment is made of animal skin and treated with lime solution that contains calcium, but the amounts of calcium showing up in the results for these colors was significantly higher than we had anticipated. The red and the gold pigments had some traces of calcium, but not nearly as much as the incredible amounts within the black and blue pigments. We cross-referenced the calcium levels in the pigments with calcium detected on the calendar’s parchment. We saw enough significant difference between the calendar pages and the pigments themselves to suggest an unusual presence of calcium in the black ink and blue pigment.
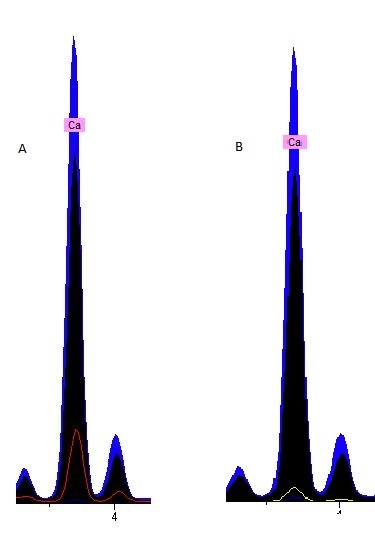
In the image above, on the left (A), the red color on the p-XRF results represents the red penwork on fol. 6r in comparison to the blue and the black pigments in their levels of calcium. We can see here that the red pigments used in the calendar pages of the Hargrett Hours have traces of calcium, but it is nothing compared to the blue (represented by blue in the above images) and the black (represented by black). Similarly, the image on the right (B) is the gold pigment on fol. 6r, represented by the yellow line, against the blue and black pigments to show an even lesser amount of calcium in the sampling spots. We believe that the small trace of calcium that is present in the gold sampling spot is evident of using gesso, as gold leaf is applied with gesso. We were left wondering why in the world the black and blue pigments have sky-rocketing levels of calcium in comparison to our reds and golds.
What We Learned
We had already suspected that the calendar was written by a different scribe than the rest of the Hargrett Hours based on the differences in handwriting, but we didn’t expect the calendar’s spectral results to look so different from the other parts of the manuscript. While the types of pigments seem to line up with the results from the rest of the manuscript, these high calcium levels found only in the calendar suggest something was done differently between these sections.
Dr. Hunt proposed a few possibilities for the high levels of calcium. The strongest two theories are centered around how the scribe stored pigments. The first theory is that the pigments with high calcium were stored in a horn inkwell. Horns are high in calcium, and so it is possible that calcium ions could have leached into pigments being stored within them. The Museum of London has several horn inkwells that were used all throughout the medieval era, so it is possible that the scribe for the calendar of the Hargrett Hours used horn inkwells.

The second theory is that the pigments were stored in glass inkwells. There are three broad categories of medieval glass: soda glass, potash glass, and high-lead glass. The differences in these types come from the alkali metal component, but all three are made of silica and lime. Because of how the p-XRF machine functions, it cannot effectively detect silica. However, lime, or calcium oxide, could be the reason for the unusually high levels of calcium in the calendar’s black and blue pigments.
If the scribe used glass inkwells, washing them out with water could have contaminated the pigments with elements from the glass. When water reacts with glass, the alkali ions (like calcium) from the glass basically switch with the water ions, leaving loose alkali ions that can leach into whatever is being stored in the glass. Additionally, medieval glass isn’t particularly durable, and the quick rate of decay – especially in potash glass – could have contributed to the unexpected elements seen in our results.

Either of these theories could suggest that there were multiple scribes that kept inks in different containers, but that wouldn’t necessarily mean that the calendar wasn’t part of the original Hargrett Hours. It should be said that we only have speculation to go on, and our theory is based on external research. Maybe future semesters of the materials analysis course can help prove our theory.
Works Referenced
Hoover, Johanna, et al. “Digging for Gold in the Hargrett Hours.” Hargrett Hours Project. 2019. https://ctlsites.uga.edu/hargretthoursproject/digging-for-gold-in-the-hargrett-hours/
ILLUMINATED, The Fitzwilliam Museum of the University of Cambridge. ww.fitzmuseum.cam.ac.uk/illuminated
MacGregor, Arthur. “Bone, Antler, and Horn: An Archaeological Perspective.” Journal of Museum Ethnography, no. 2, 1991, pp. 29–38. JSTOR, www.jstor.org/stable/40795034.
Tyson, Rachel Caroline. Medieval Glass Vessels in England AD 1200-1500 : a Survey., Durham theses, Durham University. 1996. http://etheses.dur.ac.uk/1223/
Images Used
“Inkwell.” Museum of London. https://collections.museumoflondon.org.uk/online/object/36056.html
London, British Library, Harley MS 641, fol. 118. British Library Digitized Manuscripts. http://www.bl.uk/catalogues/illuminatedmanuscripts/ILLUMIN.ASP?Size=mid&IllID=19074
University of Georgia, Hargrett Library, Hargrett MS 836. (The Hargrett Hours). Hargrett Hours Project. http://hargretthoursproject.digilabuga.org/.